The NIH funded CMI network
The Computational Models of Immunity (CMI) network was created to develop computational models of immunity to infectious diseases other than HIV/AIDS. CMI member labs are sought to develop, refine, and validate computer-based models of immune responses: (1) during or following infection, and/or (2) before and after vaccination against infectious disease through an iterative approach involving computational studies and immunological experimentation. Investigators are encouraged to develop widely generalizable models or those that focus on immunity to infectious diseases other than HIV/AIDS. This CMI network seeks to advance the understanding of the complex immune mechanisms which is further explained in Figure 1.
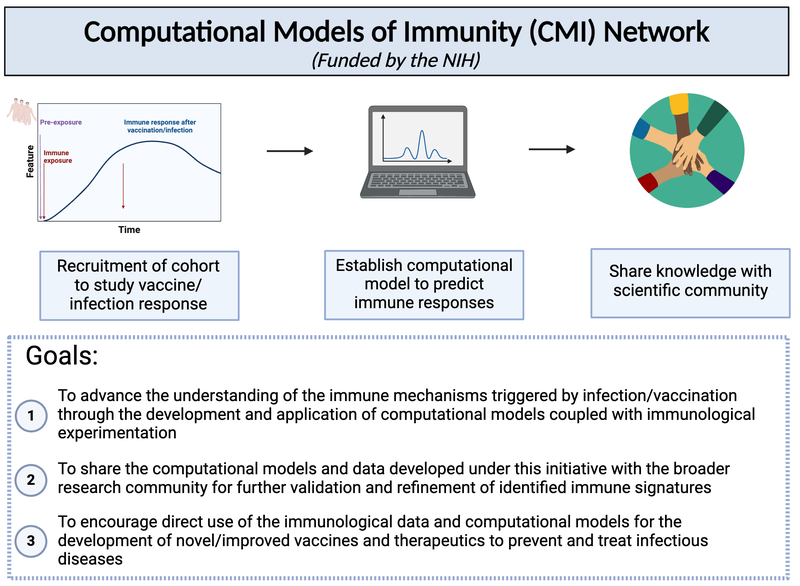
Figure 1: Outline of the CMI network.
A community prediction challenge
Computational models that predict an individual's response to a vaccine offer the potential for mechanistic insights and personalized vaccination strategies. These models are increasingly derived from systems vaccinology studies that generate immune profiles from human cohorts pre- and post-vaccination. Most of these studies involve relatively small cohorts and profile the response to a single vaccine. The ability to assess the performance of the resulting computational models would be improved by comparing their performance on independent datasets, as has been done with great success in other areas of biology, such as protein structure predictions. To transfer this approach to system vaccinology studies, we established a platform that focuses on the evaluation of Computational Models of Immunity to Pertussis Booster (CMI-PB) vaccinations [1]. A community resource, CMI-PB generates experimental data for the explicit purpose of model evaluation, which is performed through a series of annual data releases and associated challenges.
A hurdle in developing computational models for biomedical applications is objectively testing their generalizability and predictive performance[2][3]. This is especially true for systems vaccinology studies due to the complexity and variability of the assay readouts, differences in study designs, and incomplete understanding of what the clinically relevant correlates of a vaccine-induced protective response are. Integrating diverse data types, accounting for inter-individual variability, and capturing temporal dynamics are crucial aspects that need to be addressed to ensure the robustness and accuracy of computational models in system vaccinology. The CMI-PB project will attempt to address the underlying issues explained above and provide resources to develop and test computational models that predict the outcome of Tdap booster vaccination, which is designed to be used by the broader community.
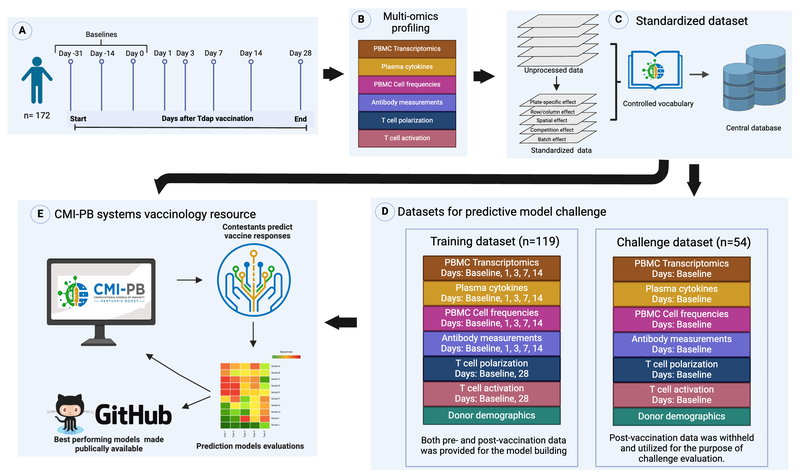
Figure 2: Outline for establishing the CMI-PB resource.
The objective of the CMI-PB prediction challenge is to foster a collaborative research community, addressing problems and advancing scientific knowledge more rapidly than any individual or research group could achieve alone. Through this website, we are organizing a yearly public challenge to predict the immune responses of individuals characterized in the previous year. The contest instructions include information on datasets generated and variables to be predicted. Each prediction team will answer a list of questions. All predictions made will be in the form of ranking readouts from the highest response (as rank 1) to the lowest response in the N donors tested (rank N). We will evaluate the answers to these questions using Spearman correlation. This will facilitate constructive discussions between modelers and experimentalists based on transparent metrics.
We have organized three annual challenges. The inaugural challenge, held in May 2022, saw active participation from the CMI-PB consortium and was a success. We are pleased to announce that the second challenge concluded in January of 2024 and participants for this challenge included not only the CMI-PB consortium, but also a group of external contestants who had been invited to participate. You can find more information on the second challenge here on the Solutions Center. We are excited to engage members of the public in the third challenge beginning on August 27, 2024.
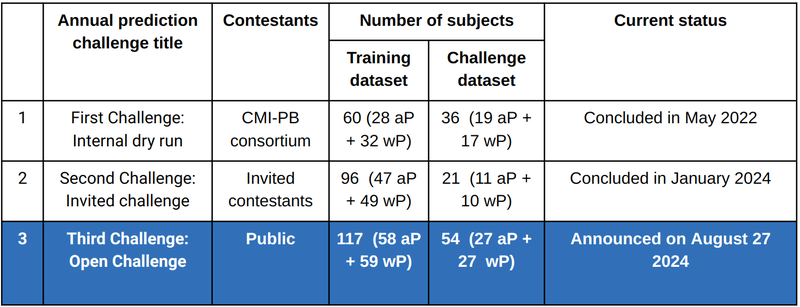
Table 1: Past, current and future CMI-PB annual prediction challenges.
For continuity and benchmarking, the first and second challenges incorporated training data from a previously published study[4] along with freshly generated challenge data. Similarly, in the upcoming challenge, we will employ both the training and challenge data from prior competitions as the foundation for training while creating new datasets specifically for testing purposes.
To find the webpages used for the past 2nd CMI-PB Challenge, please follow the links below:
- Link to Data Overview (study outline, sample and data collection, information, and data standardization)
- Link to Access the Data
- Link to General Information of the 2nd Challenge
- Link to the 2nd Challenge Solutions Center Category
Pertussis (Whooping Cough)
Pertussis, or whooping cough, is a highly contagious lung infection caused by the bacteria Bordetella pertussis. Pertussis can infect people of all ages but is most severe and life threatening for infants under a year old[5]. This is due to both the small size of their airways and because they are too young to have completed their full course of vaccinations. Transmission occurs primarily through bacteria-laden respiratory droplets produced when an infected individual coughs and sneezes[6].
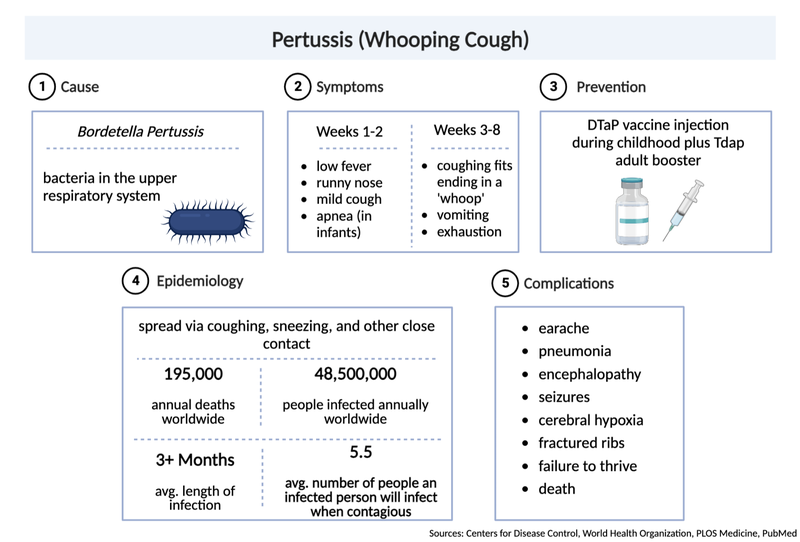
Figure 3: Pertussis (Whooping Cough).
Inhaled bacteria can then attach and colonize ciliated cells of the respiratory tract in a new host. Proliferating bacteria release a potent cocktail of toxins that both damage cilia and impede the immune response to infection (Figure 3)[7]. These damaged cilia can no longer effectively transport mucus and foreign particles out of the lungs. This leads to mucus build up and contraction of the airways leading to violent, uncontrollable coughing fits. As the cough becomes more severe it may be followed by a “whooping” sound upon inhalation. These characteristic symptoms can persist for many weeks giving rise to the common disease names of whooping cough and the hundred day cough. In this first vignette we focus largely on pertussis pathogenesis and history of control measures. This is important because, as discussed below, despite high levels of vaccination many countries are now experiencing a significant increase in pertussis cases with large outbreaks now once again a major public health concern.
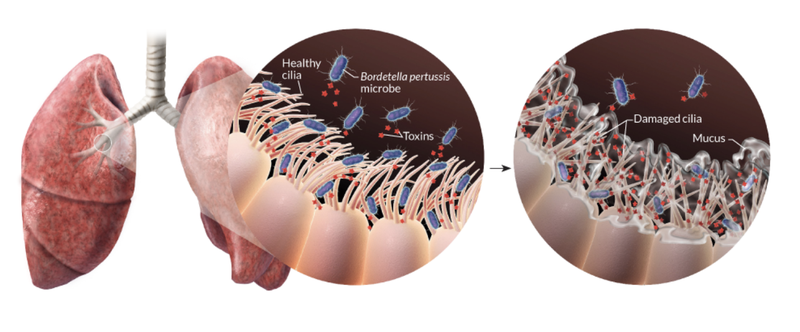
Figure 4. Bordetella pertussis attacks cells lining the airways. The rope-like structures shown are cilia, that typically sweep away inhaled dirt and foreign objects. In a pertussis infection, the bacteria use adhesive proteins (such as Filamentous hemagglutinin, Pertactin, and Fimbriae) to stick whilst releasing toxins (including Pertussis toxin, Dermonecrotic toxin and Tracheal cytotoxin) that damage cells, trigger inflammation and increase mucus production leading to uncontrollable violent coughing. Source: Nicolle R. Fuller/Science Photo Library[8]
Pertussis vaccination
Fortunately, a vaccine is now widely used to prevent the spread of whooping cough. The combination of the vaccine and booster is a powerful defense against pertussis, the nasty bacterial disease responsible for causing this highly contagious respiratory illness.
For young children, the acellular pertussis antigens (DTaP) vaccine provides protection with smaller concentrations of diphtheria toxoids and pertussis antigens. Older children, teenagers, and adults are administered the Tdap vaccine booster, which features acellular-pertussis toxoids (Figure 5).
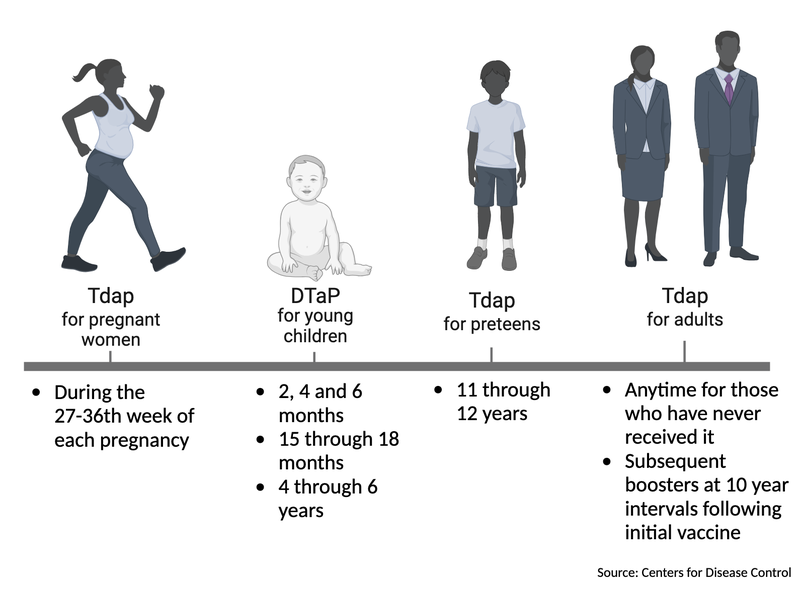
Figure 5: Pertussis vaccination and booster timeline per age group.
Before the advent of the pertussis vaccine, whooping cough was a common and often deadly scourge, with over 200,000 cases reported annually in the United States alone[9]. Fortunately, with the introduction of the whole-cell pertussis (wP) vaccine in the 1940s, the number of cases plummeted, leading to a significant decrease in the spread of the disease[10][11][12]. However, the wP vaccine was used for many decades, but concerns arose over its potential side effects[13].
In the 1990s, a new vaccine emerged based on acellular-pertussis (aP) bacteria, which caused fewer severe adverse reactions. This aP vaccine is a mixture of several purified B. pertussis antigens, providing a safer and more effective solution to prevent the spread of whooping cough[13].
However, recent studies have shown that the protection provided by the aP vaccine may wane more quickly[14][15] than the wP vaccine, leading to a resurgence of pertussis cases in multiple countries. As a result, some countries have reverted back to using the whole-cell pertussis vaccine in their immunization programs. The reason for the increase in infections has not yet been fully elucidated, but there have been several potential explanations which include mutation of the bacteria, poor vaccination rates, and asymptomatic transmission. However, these explanations have only partially been addressed and have not been supported by experimental and epidemiological data.
Annual prediction challenges
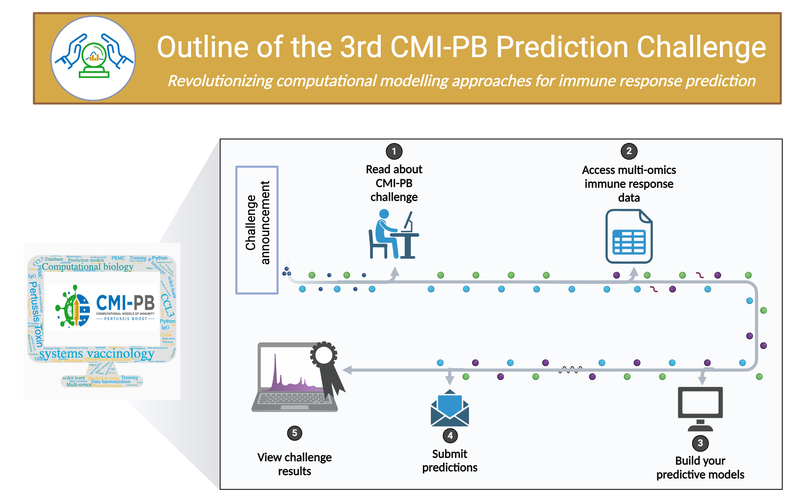
Figure 6: CMI-PB Challenge Outline.
The Community for Modeling and Pertussis Boost (CMI-PB) platform brings together a community of experts and enthusiasts to advance our understanding of the human immune system. With the CMI-PB resources, you will have access to a wealth of experimental data and the opportunity to use your expertise to help solve complex problems.
Every year, we host an open contest that challenges participants to predict the immune responses of 20+ individuals after pertussis booster vaccination. This is your chance to put your skills to the test and potentially make a groundbreaking discovery that could change the course of scientific research. Join the community, engage in discussions, and learn from the best in the field. The 3rd (Public) CMI-PB Prediction Challenge is set to begin on August 26, 2024. If you have any initial questions, please ask our team via the Solutions Center. Help us uncover the mysteries of the immune system and make a lasting impact on the world of science.

Figure 7: The team at La Jolla Institute for Immunology working to create a prediction model for the 2nd CMI-PB Challenge.